

Due to the highly reactive nature of C 2, most experimental studies involve flame-emission 17– 20 or plasma-discharge 21, 22 spectroscopy. However, not all of the recent studies are in agreement, with some research preferring the notion of a double 12, triple 3, or quasi double–triple 13 bond, whereas other studies note that the theoretical approaches are not sufficient to definitively discern between the different bonding models 14– 16.ĭespite advances in spectroscopic techniques, experimental studies have not been able to confirm any of the suggested bonding structures, with the majority of the debate currently driven by the results of ab-initio calculations 2. This high bond-order model is further supported by a subsequent quantum chemistry calculation, which found higher magnetic shielding in C 2 compared with C 2H 2, supporting a bulkier C–C bond 11. The strength of this inverted bond between the s p 1 orbitals has since been calculated at various levels of theory and is estimated to contribute ~50–80 k mol −1 1, 5, 9, 10.
C2 molecular orbital diagram plus#
This included contributions from a σ bond, 2 × π bonds, plus an interaction between the outward pointing s p 1 hybrid orbitals 1.
C2 molecular orbital diagram full#
A recent high-level full configuration–interaction calculation, combined with valence bond (VB) theory, identified four distinct contributions to the bonding in C 2 1. However, if molecular orbital (MO) theory is used, a ground-state valence electron configuration for C 2 of KK ( σ 2 s ) 2 ( σ 2 s * ) 2 ( π 2 p ) 4 is predicted, yielding a bond order of 2, with the unusual situation of a π double bond with no accompanying σ bond.įrom an ab-initio approach, standard Hartree–Fock-based calculations support a dicarbon double bond however, more advanced theoretical studies have suggested that the C–C bond may be better described by a higher bond order 5– 8.
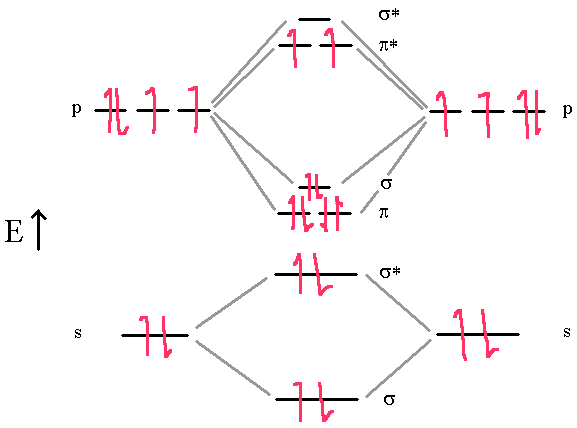
In contrast, a hybrid-orbital (HO) style approach invokes s p 1 hybridisation to predict a triple bond between the carbons. However, this bonding assignment seems unlikely, with stable quadruple bonds typically only found between transition metal elements that have partially filled d-orbitals 4. From a simple Lewis electron-pair repulsion description, the 8 valence electrons in C 2 are predicted to form a quadruple bond. Standard qualitative theories predict different values for the dicarbon bond order 2, 3. This discussion has been driven by recent advances in computational methods, with various studies suggesting the carbon–carbon bond may have a bond order of 2, 3, or even 4, with the latter from ab-initio studies 1. Each non-bonding pair is distributed over both oxygen atoms at once in molecular orbital theory, while in Lewis theory each lone pair is isolated to one atom or to localized bonds attached to that atom.Despite the relative simplicity of homonuclear diatomic molecules, the bonding nature of dicarbon, C 2, has long been a topic of debate. Still, notice that each orbital is spread across both oxygen atoms at once, and again we see that each non-bonding electron pair in the HOMO is very different in molecular orbital theory compared to Lewis theory. \( \newcommand\) molecular orbitals, which are truly non-bonding and mostly oxygen in character.


 0 kommentar(er)
0 kommentar(er)
